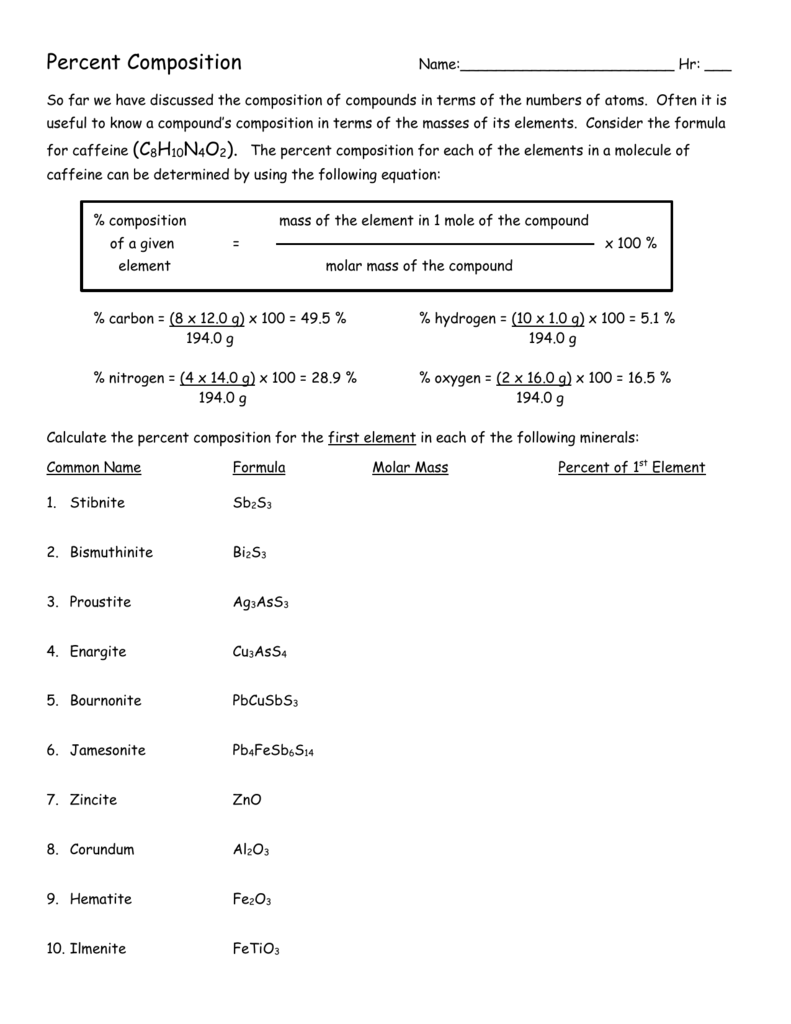
Find the Percent Composition of Each Element in Caffeine C8h10n4o2
1 mol C 7569 g C x 6302 mol C 1201 g C 1 mol O 09694 mol O 1551 g O x 1600 g O 1 mol H 880 g H x 871 mol H 101 g H C6302H871O09694 divide each subscript by the smallest 09694. Explain how you can find the mole ratio in a chemical compound.

Aim How Do I Calculate Percent Composition Ppt Download
Caffeine has the following composition.

. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Multiply each subscript by 2. 4948 of carbon 519 of hydrogen 1648 of oxygen and 2885 of nitrogen.
Molar mass of C8H10N4O2 1941906 gmol. Convert grams C8H10N4O2 to moles or moles C8H10N4O2 to grams. Hydrogen 19419 x 00519.
1201078 10079410 1400674 1599942 Percent composition by element. Carbon 19419 x 04948 960852. This compound is also known as Caffeine.
Percent composition is numerically equal to the mass in grams of each element in a 1000 g sample. The mole ratio is determined by calculating the moles of each element in the compound and dividing each number of moles by the smallest number of moles. How many grams of KMnO4 correspond to 313 x 1022 formula units of.
- The atomic mass of each element contained in the compound - The formula of the compound. Academiaedu is a platform for academics to share research papers. Find out the molecular and empirical formula.
C8H10N4O2 molecular weight. CCI4 - 1538 gmol CH2O - 3003 gmol SF6 - 1461 gmol KrF2 - 1218 gmol. Multiply percent composition with the molecular weight.
C6302 09694H871 09694O09694 09694 C65H9O. Associate each molar mass with its compound CCI4 - 3003 gmol CH2O - 1218 gmol SF6 - 1538 gmol KrF2 - 1461 gmol. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry.
The molecular weight is 19419 gmol. It is sometimes necessary. From the percent composition data a 1000 g sample contains 7569 g C 1551 g O and 880 g H.

Composition Of Substances And Solutions Ppt Download

Empirical Molecular Formula Of Caffeine From Composition Data Youtube

Comments
Post a Comment